- Startseite
- entfernung membran
- Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions - ScienceDirect
Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions - ScienceDirect
4.7 (504) · € 17.99 · Auf Lager

Cryo-EM-based structural insights into supramolecular assemblies of γ- hemolysin from S. aureus reveal the pore formation mechanism - ScienceDirect

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect

Estimation of pore dimensions in lipid membranes induced by peptides and other biomolecules: A review - ScienceDirect
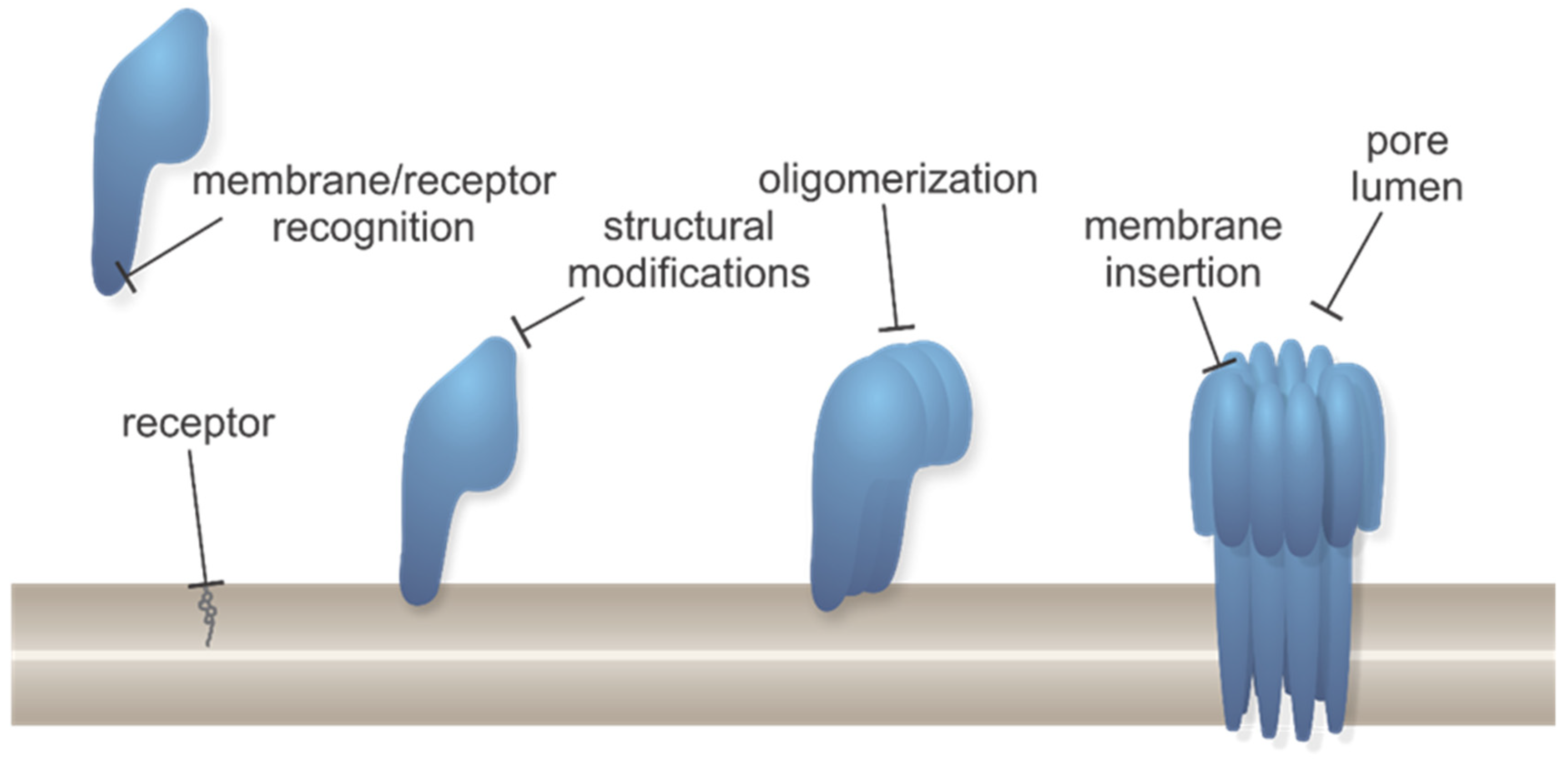
Toxins, Free Full-Text
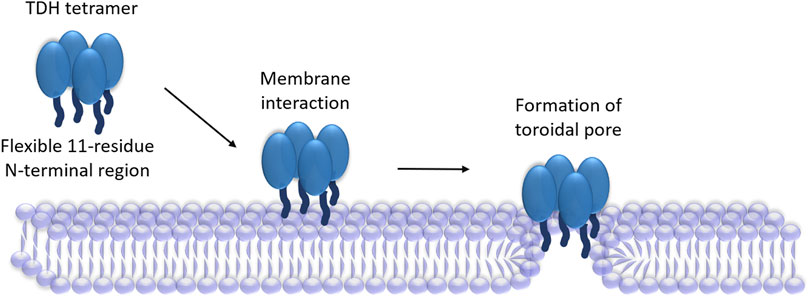
Frontiers Current Perspective on the Membrane-Damaging Action of Thermostable Direct Hemolysin, an Atypical Bacterial Pore-forming Toxin
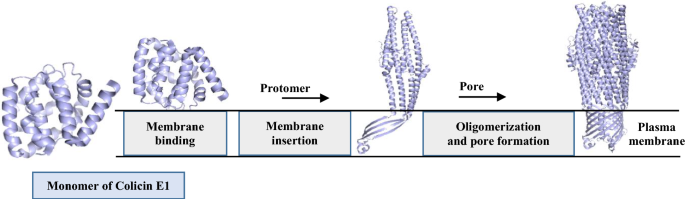
Story of Pore-Forming Proteins from Deadly Disease-Causing Agents to Modern Applications with Evolutionary Significance

Membrane pore formation at protein–lipid interfaces: Trends in Biochemical Sciences

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect

Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation

Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions

Staphylococcus aureus-Derived α-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution - ScienceDirect

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect
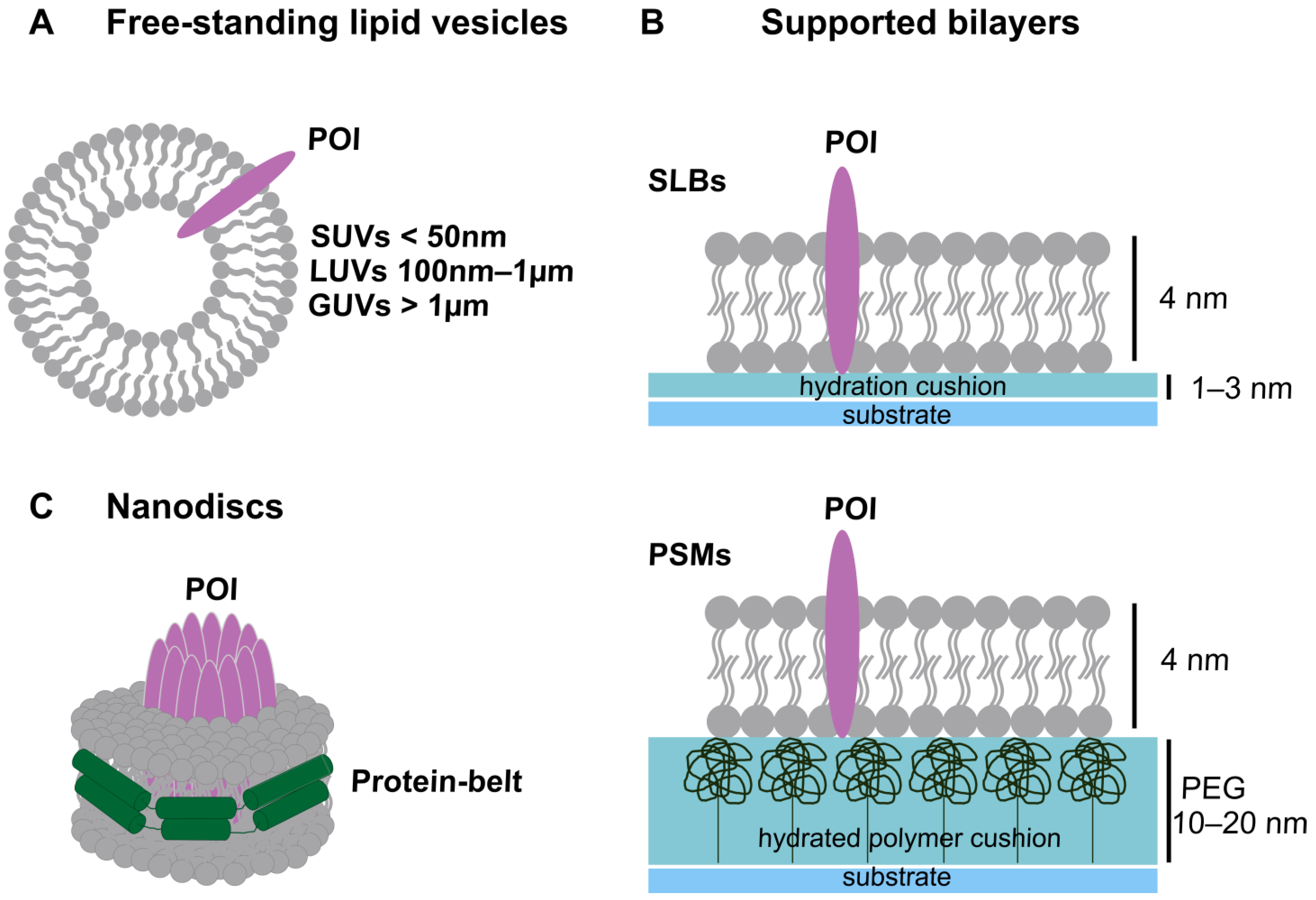
IJMS, Free Full-Text