Solved overall reaction: 2NO(g)+O2(g)→2NO2(g) Step
4.8 (92) · € 24.99 · Auf Lager
At 364 K, the equilibrium constant for the reaction: 2NO(g) ↔ N2(g) + O2(g) is Kc = 0.00307. If the initial concentration of NO is 0.153 M, what is the equilibrium concentration

How can you determine the law of the following reaction? `2NO(g) + O_(2) (g) to 2NO_(2) (g)`
For the reaction 2NO2(g) ⇋ 2NO(g) + O2(g) (Kc = 1.8 × 10^–6 at 184°C) - Sarthaks eConnect
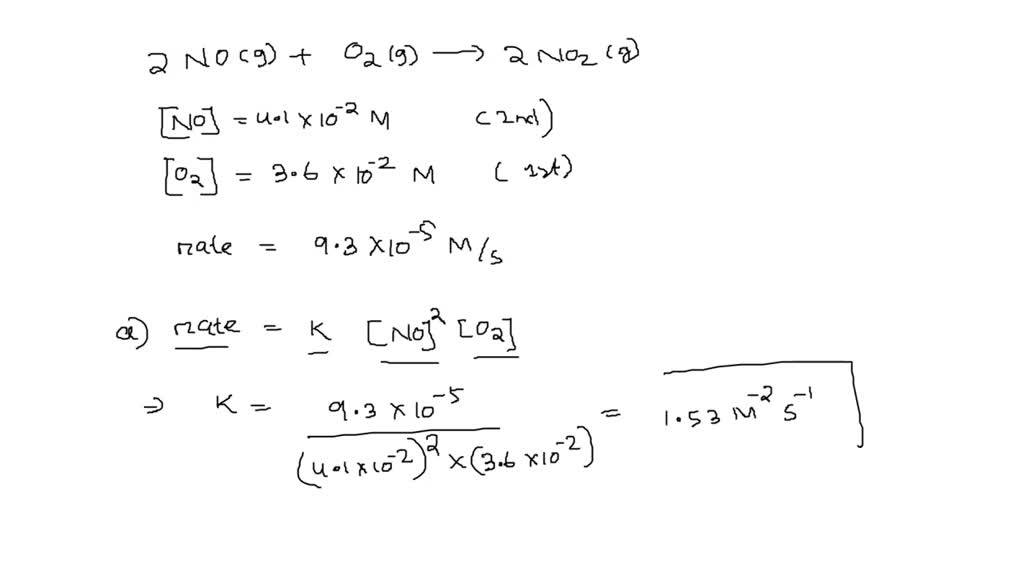
SOLVED: The reaction 2NO(g) + O2(g) â†' 2NO2(g) is second order in NO and first order in O2. When [NO] = 4.1×10^-2 M and [O2] = 3.6×10^-2 M, the observed rate of

For the reaction 2NO(g)+O2( g)→2NO2( g) calculate ΔG at 700 K when enth..

29 For reaction, 2NO(g)+O2( g)⟶2NO2( g) Rate law is : rate of reaction ..

Kinetics. - ppt download
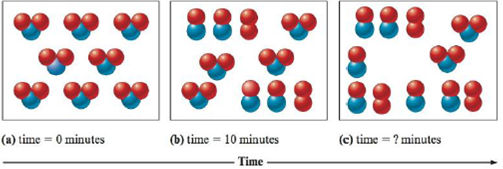
Consider the following representation of the reaction 2NO 2 ( g ) → 2NO( g ) + O 2 ( g ) . Determine the time for the final representation above if

2NO→ N2O2 (slow) N2O2 + O2→ 2NO2 (fast) For the preceding multi - step reaction mechanism, which of the following is the correct rate law?