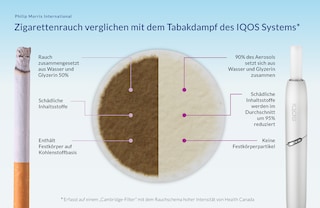
FDA Allows PM to Market IQOS as 'Reduced Risk' - Tobacco Asia
4.5 (615) · € 26.50 · Auf Lager

FDA Says IQOS Tobacco Products Reduce Exposure to Harmful Chemicals Found In Cigarette Smoke - Reason Foundation

FDA Says PM USA Can Market IQOS as Modified Risk Tobacco Products

Newer Nicotine and Tobacco Products: Philip Morris International - TobaccoTactics

Impact of different health warning label and reduced exposure messages in IQOS ads on perceptions among US and Israeli adults - ScienceDirect
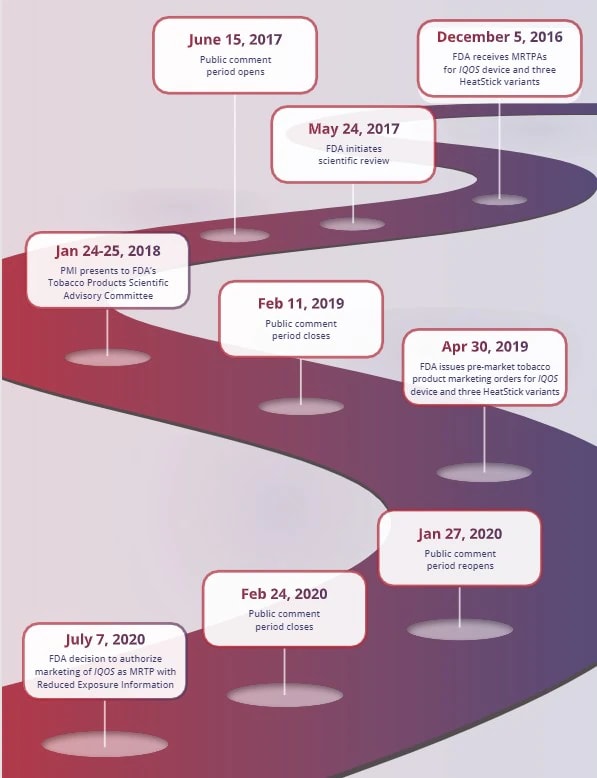
Historic decision made as IQOS Tobacco Heating System is authorized under U.S. MRTP pathway

FDA Says PM USA Can Market IQOS as Modified Risk Tobacco Products

Altria Tobacco Asia

F.D.A. Panel Rejects Philip Morris' Claim That Tobacco Stick Is Safer Than Cigarettes - The New York Times

FDA authorizes IQOS as MRTP Philip Morris International

FDA approves sale of Philip Morris IQOS heated tobacco devices

PMI to Regain Full U.S. Commercial Rights to IQOS

Philip Morris sees six million U.S. smokers switching to iQOS device if cleared - Georgia Asian Times

Big Tobacco Has a Lot Riding on FDA's Stance Toward IQOS, the Latest Cigarette Alternative - WSJ






:focal(465x240:475x230)/cloudfront-eu-central-1.images.arcpublishing.com/ipmgroup/TAOO4P5HEVDNRI67I7AR54YCKE.jpg)